Abstract
Background Patients (pts) of minority race or ethnicity are often underrepresented in oncology clinical trials (Awidi et al. JCO Oncol Pract. 2021) despite differences in the incidence of most cancer types, including B-cell malignancies, across races and ethnicities (Teras et al. CA Cancer J Clin 2016; Feng et al. Am J Epidemiol 2021). Both clinical trials and real-world (RW) studies have demonstrated significant clinical benefits of CAR T-cell therapy for pts with B-cell malignancies. To understand potential underrepresentation of minority pts, we conducted a series of simulations to estimate proportions of minority pts potentially eligible for either CAR T-cell trials or receiving standard-of-care CAR T-cell therapy in RW settings. We also assessed whether proximity to a CAR T-cell ATC is a barrier for treatment.
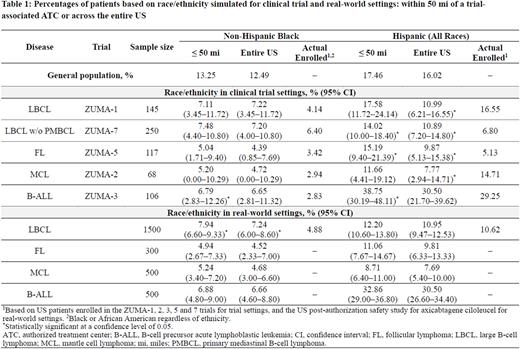
Methods Race/ethnicity demographics (mutually exclusive groups of non-Hispanic White [NHW], non-Hispanic Black [NHB], non-Hispanic Other [NHO], and Hispanic [any race]) for US pts ≥ 20 years old with large B-cell lymphoma (LBCL), follicular lymphoma (FL), mantle cell lymphoma (MCL), and B-cell precursor acute lymphoblastic leukemia (B-ALL) were estimated for both trial and RW settings based on 5-year limited-duration prevalence prior to January 1, 2019 from the SEER Research Plus Data (17 registries, November 2021 submission), and US County Population Data (1969-2020). Estimated proportions and 95% confidence intervals (CIs) were simulated based on 3000 random samples per cancer type. Sample sizes for trial settings corresponded to US enrollment in clinical trials of axicabtagene ciloleucel (axi-cel) and brexucabtagene autoleucel (brexu-cel), while those for RW settings were based on accrual targets for post-authorization safety studies (PASS) using the CIBMTR registry (Table). Sampling was performed for counties within 50 miles [mi] of trial-associated ATCs or commercial ATCs for trial or RW settings, and for the entire US.
Results As of January 1, 2019, approximately 177 million of 246 million people (72%) ≥ 20 years old lived within 50 mi of a commercial ATC in the US, of which 13% were NHB and 17% were Hispanic (Table).
In clinical trial settings, the estimated proportion of NHB pts ≤ 50 mi of an ATC ranged from 5% in FL to 7% in LBCL (Table). The estimates were numerically higher, but not statistically significant, compared with actual enrollment in trials, except in B-ALL (7% [3%-12%] estimated vs 3% enrolled). Compared with actual trial enrollment, the estimated proportions (95% CI) of Hispanic pts were statistically higher across all indications (LBCL, 14% [10%-18%] estimated vs 7% enrolled; FL, 15% [9%-21%] estimated vs 5% enrolled; and B-ALL, 39% [30%-48%] estimated vs 29% enrolled) except in MCL (12% [4%-19%] estimated vs 15% enrolled).
In RW settings, the estimated proportions of NHB pts were comparable to those in trial settings across all indications, whereas estimates of Hispanic pts were numerically lower (Table). The reported proportion of NHB pts with LBCL in PASS (5%) was statistically lower than the estimated proportion (8% [95% CI 7%-9%]).
To further assess the impact of proximity to ATCs, the estimated proportions were compared between regions ≤ 50 mi of an ATC and the entire US. Proportions of NHB pts ≤ 50 mi of an ATC across all disease types were comparable with the estimates of the entire US, regardless of trial or RW settings (Table). While for Hispanic pts, the estimated proportions ≤ 50 mi of an ATC were numerically higher compared with the estimates of the entire US; but the estimates were comparable in RW settings.
Conclusions This is the first simulation study to estimate the proportions of minority pts by considering disease prevalence and proximity to CAR T-cell ATCs. The possibility of patient underrepresentation is raised as the actual enrolled proportions of minority pts with certain B-cell malignancies were numerically lower than simulation-based estimates, even though most findings were not statistically significant. Based on the simulation, the estimated proportions of minority pts were comparable or even higher in regions close to ATCs indicating that proximity to ATCs may not be a key barrier for trial participation and accessibility of care. Further investigation into other factors influencing diversity in trials and accessibility of state-of-the-art cancer care in RW settings is warranted.
MG and Z-HH contributed equally.
Disclosures
Hu:Kite, a Gilead Company: Current Employment. Siddiqi:TG Therapeutics: Research Funding; Oncternal: Research Funding; Seattle Genetics: Speakers Bureau; Janssen: Speakers Bureau; PCYC: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Kite, a Gilead Company: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Juno: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; BeiGene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Pasquini:Bristol Myers Squibb: Consultancy, Research Funding; Novartis: Research Funding; Janssen: Research Funding; Kite: Research Funding. Shahani:Amgen: Speakers Bureau; Kite, a Gilead Company: Current Employment, Current holder of stock options in a privately-held company; City of Hope: Other: NA; American College of Obstetrics and Gynecology: Consultancy. Baro:Kite, a Gilead Company: Consultancy, Current Employment, Current holder of stock options in a privately-held company, Research Funding. Xie:Kite, a Gilead Company: Current Employment. Song:Gilead Sciences: Current holder of stock options in a privately-held company, Patents & Royalties; Kite, a Gilead Company: Current Employment, Patents & Royalties: Gilead. Sun:Kite, a Gilead Company: Current Employment. Xu:Kite, a Gilead Company: Current Employment. Locke:Society for Immunotherapy of Cancer: Other: Education or editorial activity; ASH: Other: Education or editorial activity; Clinical Care Options Oncology: Other: Education or editorial activity; Imedex: Other: Education or editorial activity; BMS: Research Funding; CERo Therapeutics: Research Funding; ), National Cancer Institute: Research Funding; Leukemia and Lymphoma Society: Research Funding; Aptitude Health: Other: Education or editorial activity; Daiichi Sankyo: Consultancy; Sana: Consultancy; Takeda: Consultancy; CAREducation: Other: Education or editorial activity; BioPharm Communications: Other: Education or editorial activity; A2: Consultancy; Celgene: Consultancy; Other: Patents & Royalties: patents, royalties, other intellectual property from several patents held by the institution in my name (unlicensed) in the field of cellular immunotherapy.; Wugen: Consultancy; Umoja: Consultancy; Novartis: Consultancy, Research Funding; Legend Biotech: Consultancy; Kite, a Gilead Company: Consultancy, Research Funding; Janssen: Consultancy; Iovance: Consultancy; GammaDelta Therapeutics: Consultancy; Emerging Therapy Solutions Gerson Lehrman Group: Consultancy; EcoR1: Consultancy; Cowen: Consultancy; Calibr: Consultancy; Cellular Biomedicine Group: Consultancy; Bristol Myers Squibb/Celgene: Consultancy; Bluebird Bio: Consultancy, Research Funding; Allogene: Consultancy, Research Funding; Amgen: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal